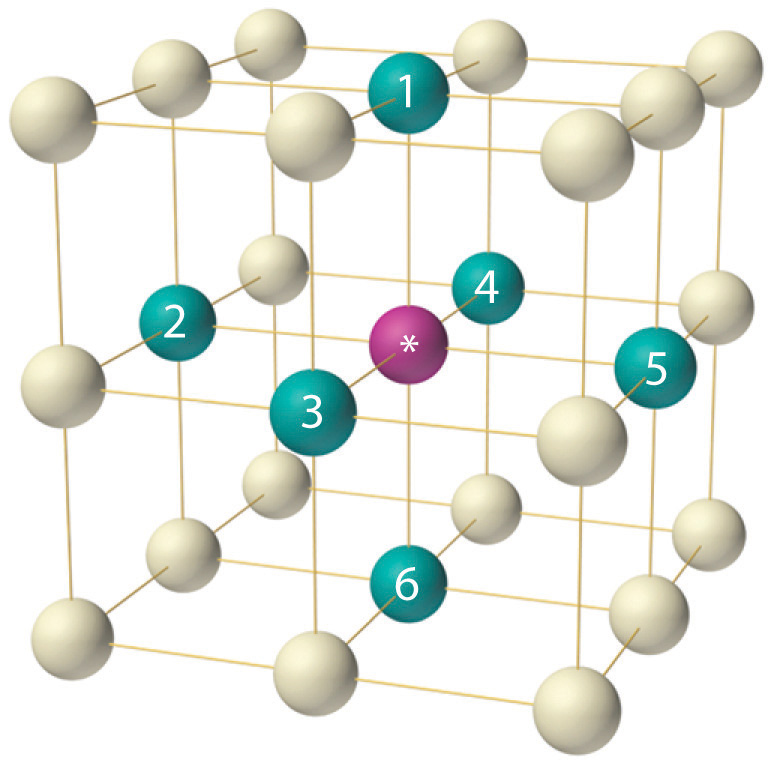
Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube
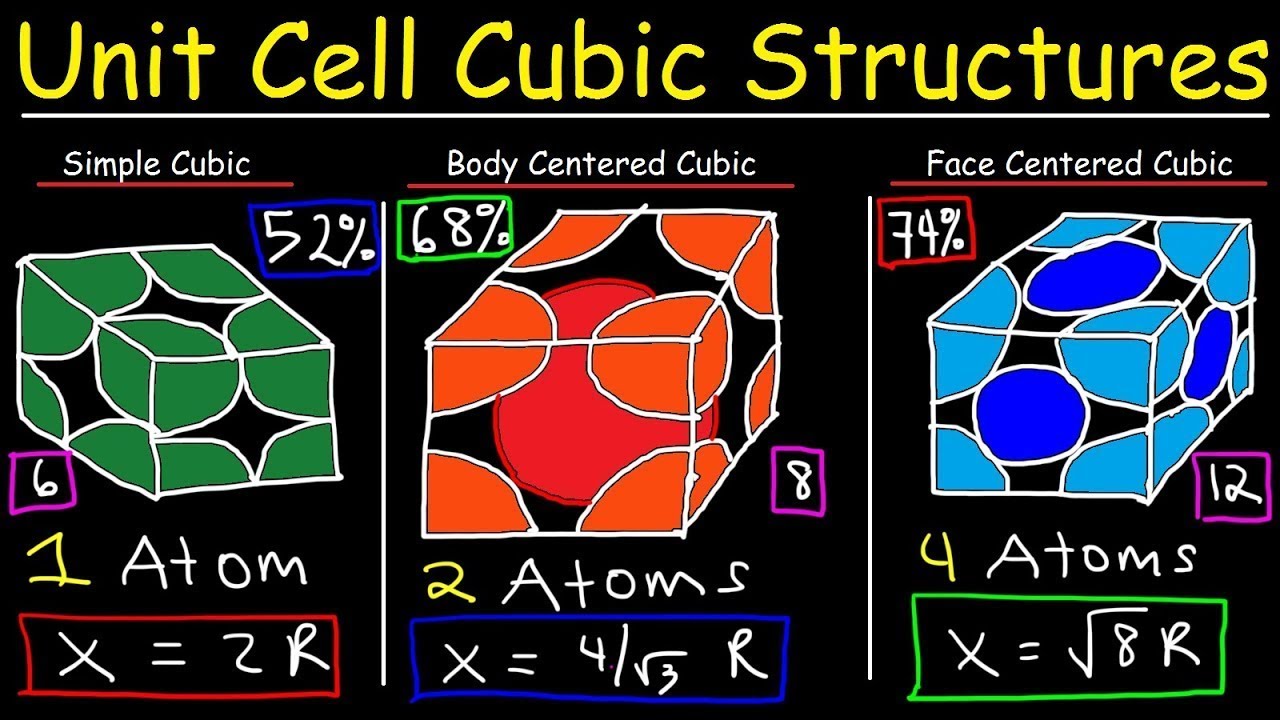
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube

Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube

An element has a body-centered cubic (bcc) structure with a cell edge of 288pm. The density...... - YouTube

HOW TO SOLVE THE EDGE LENGTH OF A FACE-CENTERED CUBIC (FCC) UNIT CELL | WITH PRACTICE PROBLEMS - YouTube

Silver crystallises in a face - centred cubic in cell. The density of Ag is 10.5 g cm^-3 . Calculate the edge length of the unit cell.

Chemistry - Liquids and Solids (27 of 59) Crystal Structure: Density of the Unit Cell: Body Centered - YouTube

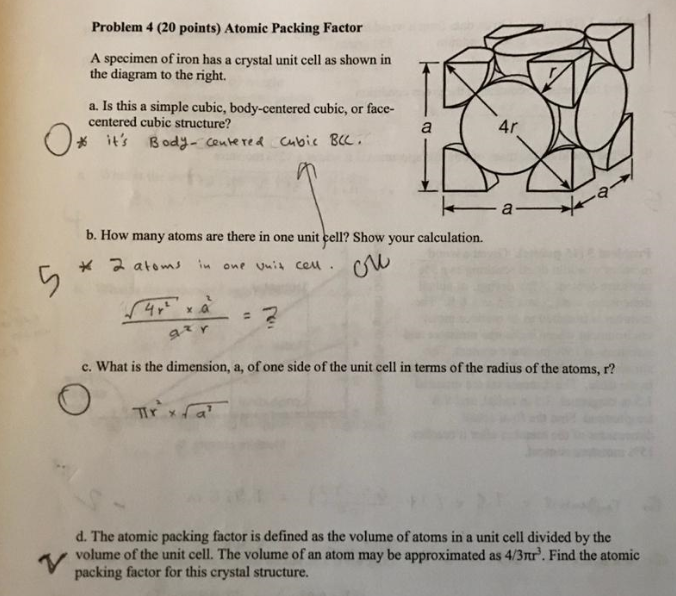
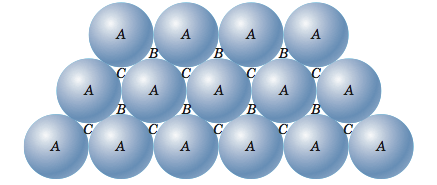

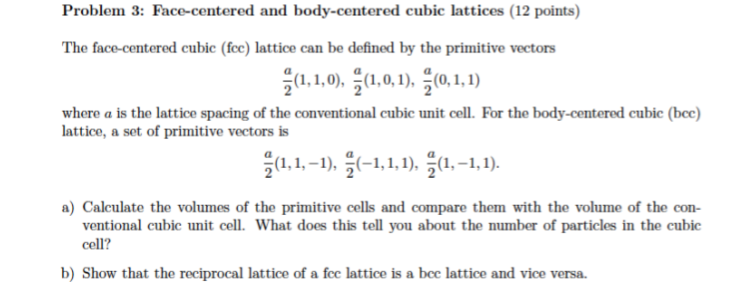
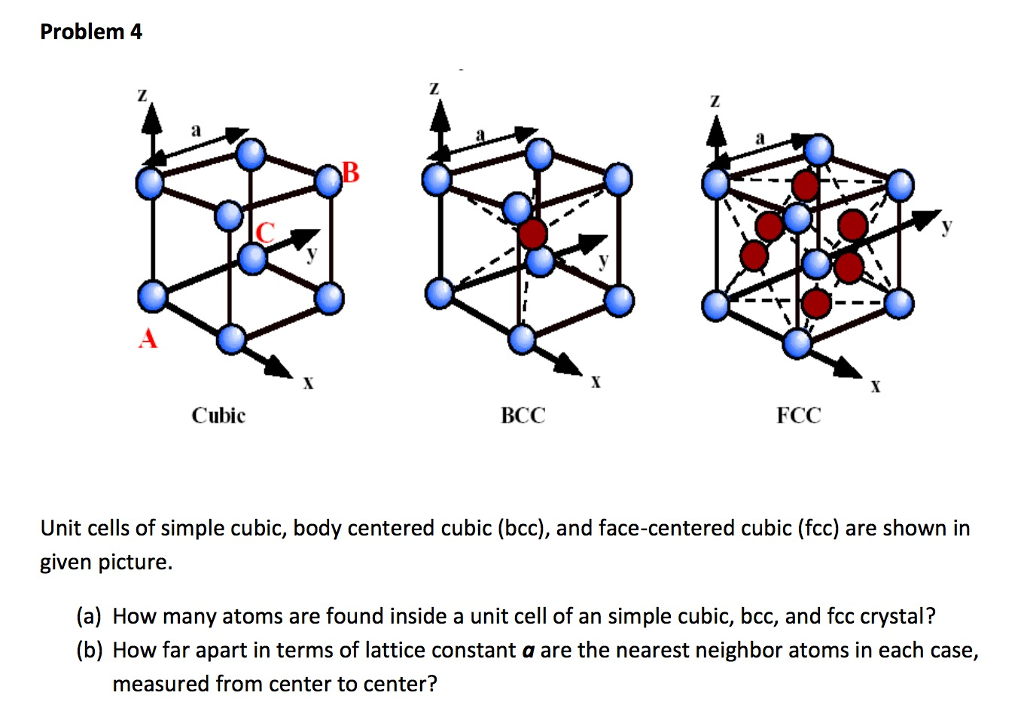



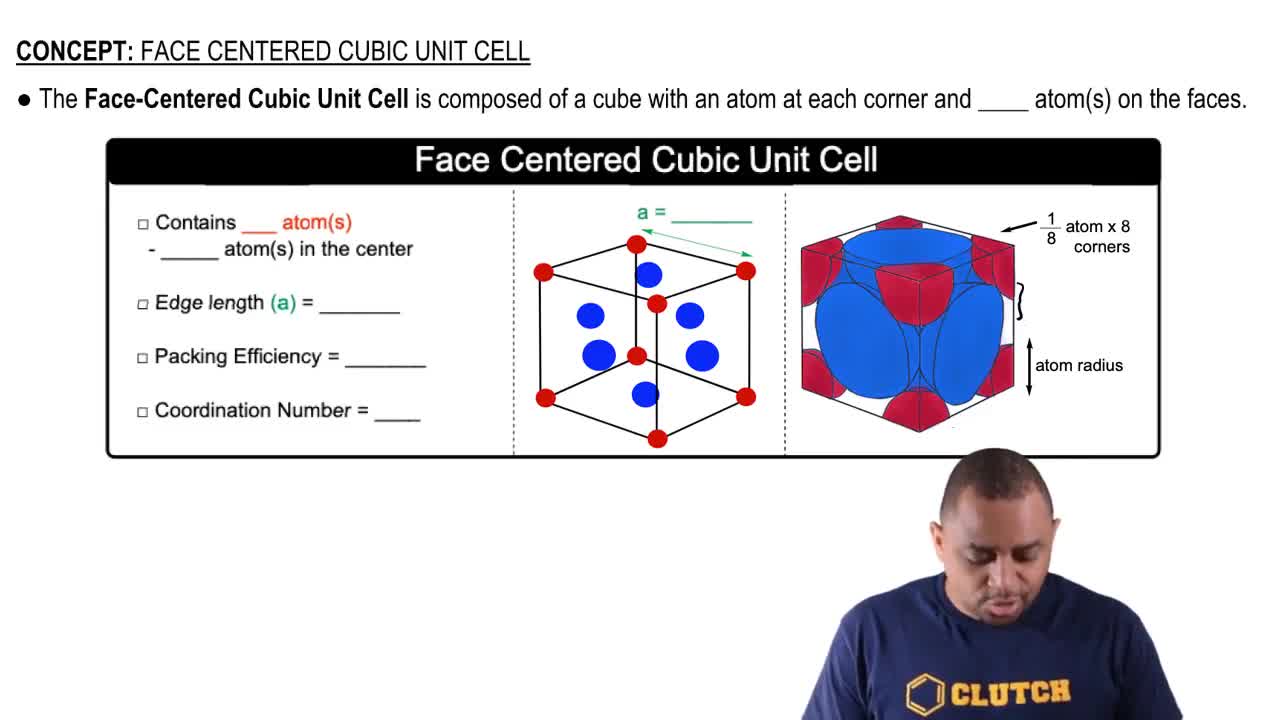
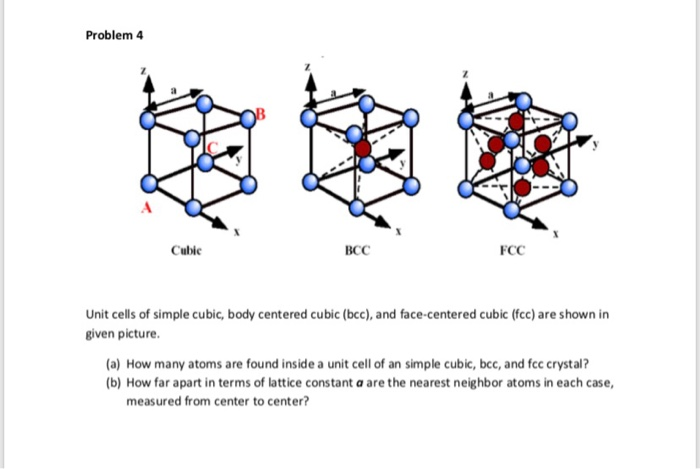
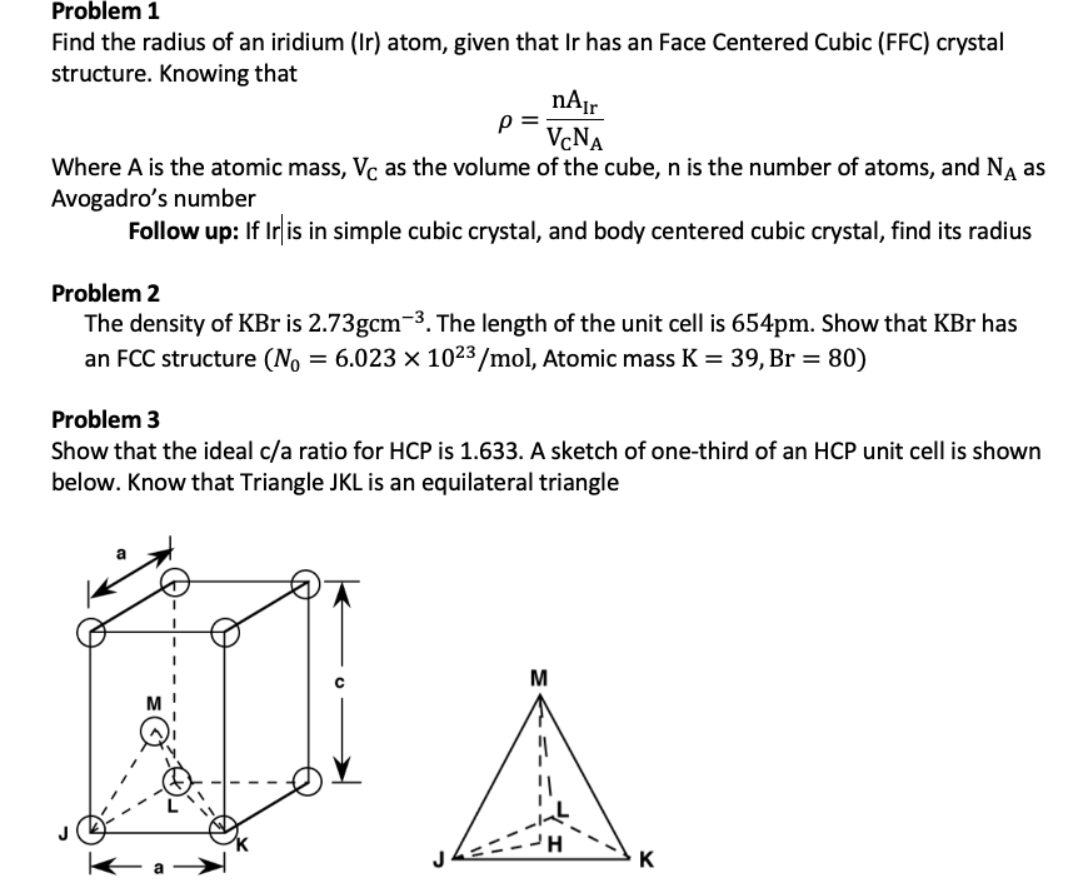
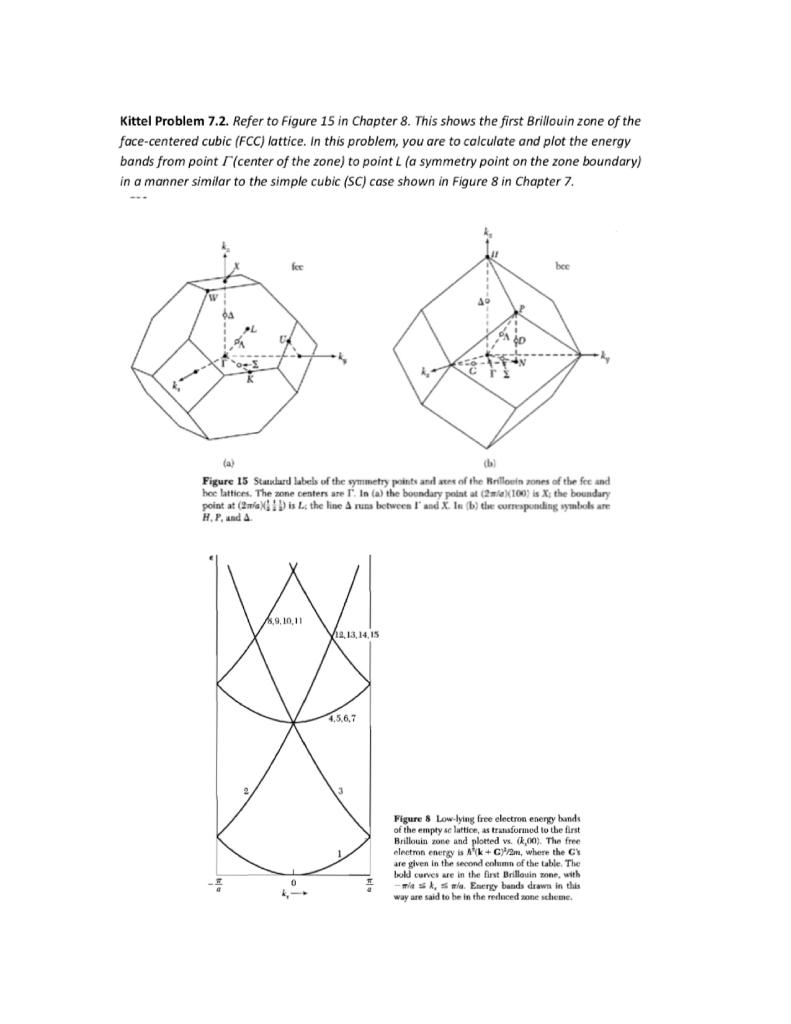

![Solved 3. Face Centered Cubic Structure [10 pts] Platinum is | Chegg.com Solved 3. Face Centered Cubic Structure [10 pts] Platinum is | Chegg.com](https://media.cheggcdn.com/media/1eb/1eb9fa27-1dc7-4453-878c-a7f39a79cbf4/phppeImhN.png)